Collaborative research on proteostasis: a look back and ahead
If we want to unlock the secrets of life, we need to look deep into the cells of our body. Every second, they are buzzing with activity: countless proteins are being created, altered and degraded, they interact with each other and are transported within the cell. Keeping all these processes in balance is essential for keeping the cells – and thus the organism – healthy and alive. But how does that work? How is the activity of proteins in a cell regulated? And how are the individual regulatory pathways interconnected in terms of time and space? Questions like these are at the heart of the comparatively young field of proteostasis research, which was officially established after the publication of an article in 2008.
The Collaborative Research Centre (SFB 969) "Chemical and Biological Principles of Cellular Proteostasis", that kicked off at the University of Konstanz in 2012 and will end this December, was the first large research consortium in Germany dedicated to studying proteostasis. From the start, scientists at the SFB have thus been able to shape the development of this young field of research. One decisive advantage the University of Konstanz had then, and still has today: the exceptionally close interdisciplinary cooperation between biologists and chemists.

"At the University of Konstanz, we recognized early on that great opportunities arise when chemists and biologists work together across disciplinary boundaries. When the field of proteostasis research emerged, Konstanz researchers already had a long track record of interdisciplinary collaboration. We were therefore optimally prepared to tackle the challenges of this new research field and were able to get started right away."
Andreas Marx, professor in the Department of Chemistry
Andreas Marx is one of the significant researchers involved in the SFB. Marx was also the speaker for the Konstanz Research School Chemical Biology, which was funded from 2007 to 2019 through the German Excellence Initiative and laid a sound foundation for the Konstanz SFB to build on. For Elke Deuerling, biology professor and speaker of SFB 969, the "Konstanz team spirit", as she calls it, was a decisive prerequisite for tackling the complex issues of proteostasis research at an internationally competitive level:
© Inka Reiter"The fact that our chemists and biologists joined forces to develop, for example, completely new methods and chemical approaches that made it possible to study certain biological processes of proteostasis in the first place, was clearly our unique selling point in Germany and perhaps even worldwide at the time."
Elke Deuerling, professor in the Department of Biology
Ubiquitous yet largely unexplored
Synthetic ubiquitin chains are one of the chemical tools that chemists and biologists led by Andreas Marx, Martin Scheffner and Florian Stengel developed within the framework of the SFB. Scheffner explains the general importance of the protein: "Ubiquitin is a small protein that – as its name says – is ubiquitous in our cells. It marks other proteins by binding them, for example to switch off or switch over their cellular function". Both technically and biologically, the ability to create specific ubiquitin chains on demand was a huge advance that the scientists could use directly for further research. They were thus able to successfully identify unknown interaction partners of the synthesized ubiquitin chains and uncover aspects of protein modification that were previously out of reach.
Ubiquitins are not only linked to each other and to other proteins in the cell. There have to be mechanisms to separate and cleave off ubiquitins in order to ensure their function in regulating various cellular processes. SFB 969's interdisciplinary team of researchers, led by Michael Kovermann, Karin Hauser, Christine Peter and Erika Isono, was able to uncover new mechanisms of ubiquitin cleavage from its substrates. "We were able to show that there are membrane-bound enzymes in the plant cell that cleave ubiquitin from its substrate and thus regulate the transport of other membrane proteins, for example, to respond to environmental stimuli. In addition, our analyses enabled us to develop a model of how the activation of these enzymes is controlled", says Erika Isono from the Department of Biology at the University of Konstanz, summarizing the results of one of the SFB studies.
Digging into the causes first
While linkage to and cleavage of ubiquitins is one of the most common protein modifications, there are numerous other modifications and factors that can control and switch the activity of proteins in the cell. If one of these regulatory mechanisms malfunctions or becomes unbalanced, this can have catastrophic consequences for the cell and the entire organism: Numerous illnesses, for example cancer or neurodegenerative diseases, result from misregulation of the cellular protein balance.
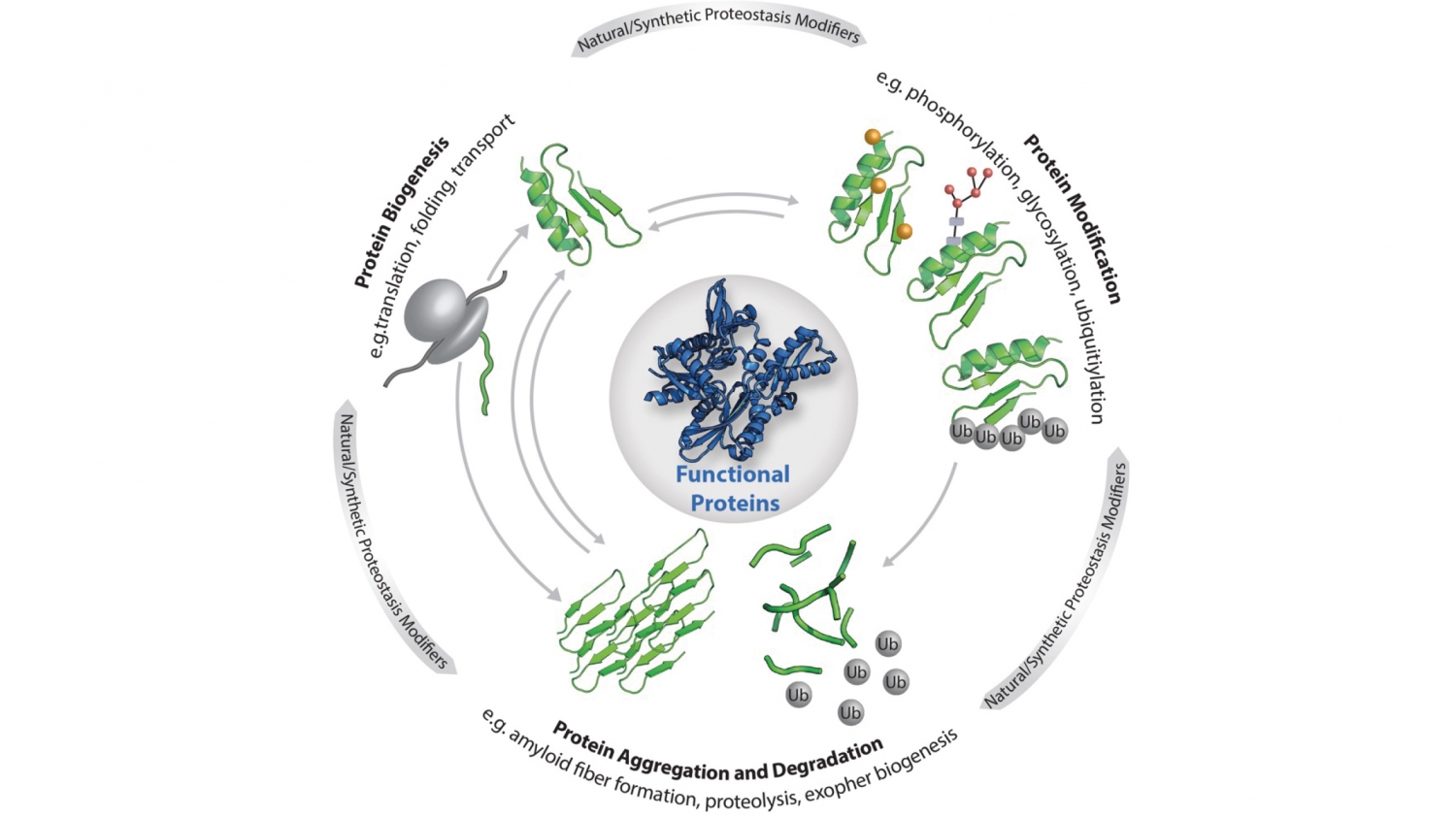
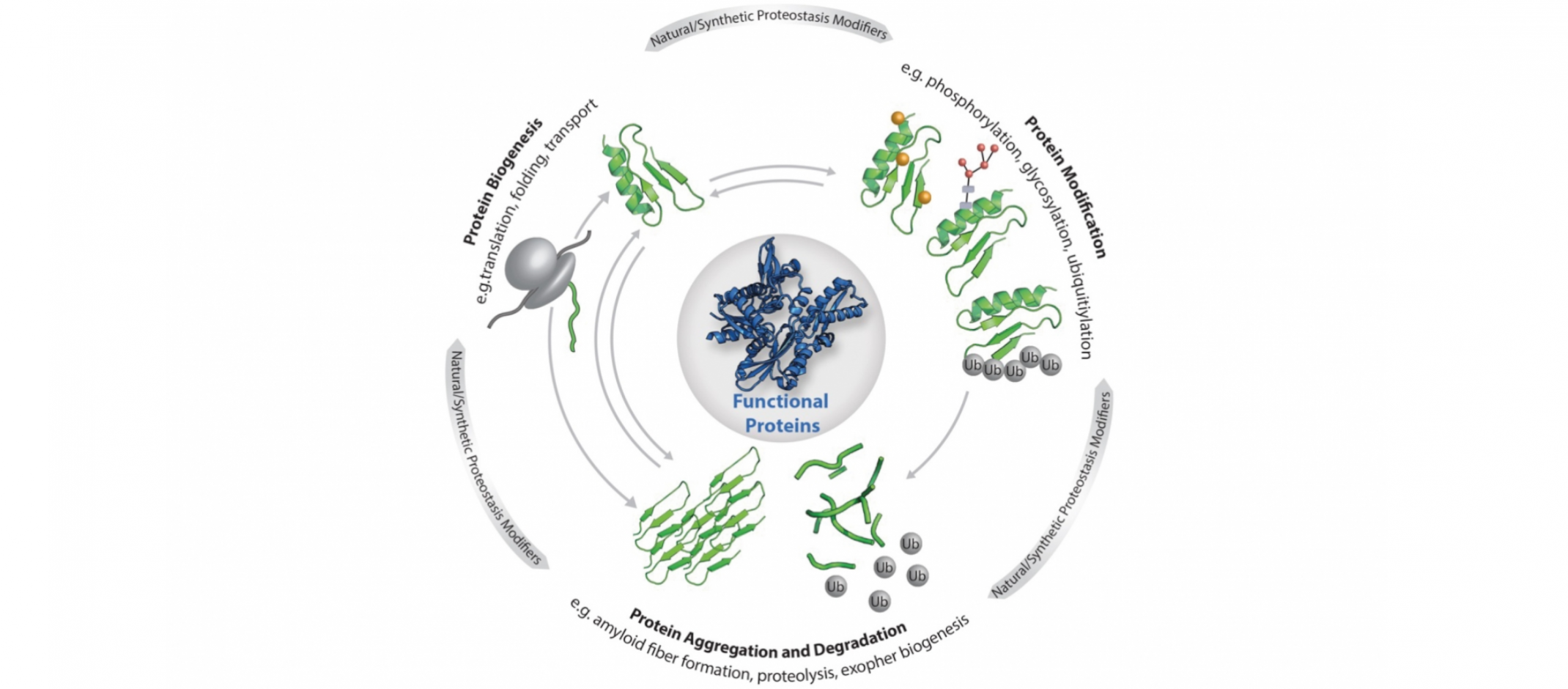
© Elke DeuerlingCellular proteostasis: The functionality of the individual proteins that make up the proteome is regulated by the central processes as shown (biogenesis, modification, degradation and aggregation). Proteostasis is also influenced by stress, ageing, metabolites as well as custom-made ligands, small molecules and artificial protein modifications.
The research of the Konstanz scientists will thus help to better understand how such diseases emerge and make it possible to develop corresponding therapies. Deuerling explains: "It is only when we understand how the cell itself functions and how its proteins are activated and regulated that we can develop approaches to address the imbalances in proteostasis that cause illness. That is why our Collaborative Research Centre is primarily doing basic research – we want to understand the underlying processes. Finding applications, of course, is also a goal, but that is the next step".
The control centre of our protein factories
In the context of the SFB, Deuerling's research team has also been able to make groundbreaking discoveries in the field of proteostasis research. With Martin Gamerdinger, Florian Stengel and other colleagues, she discovered, for example, a protein complex that serves as a control hub at the ribosomes – the protein factories of our cells – the "nascent polypeptide-associated complex", NAC for short.
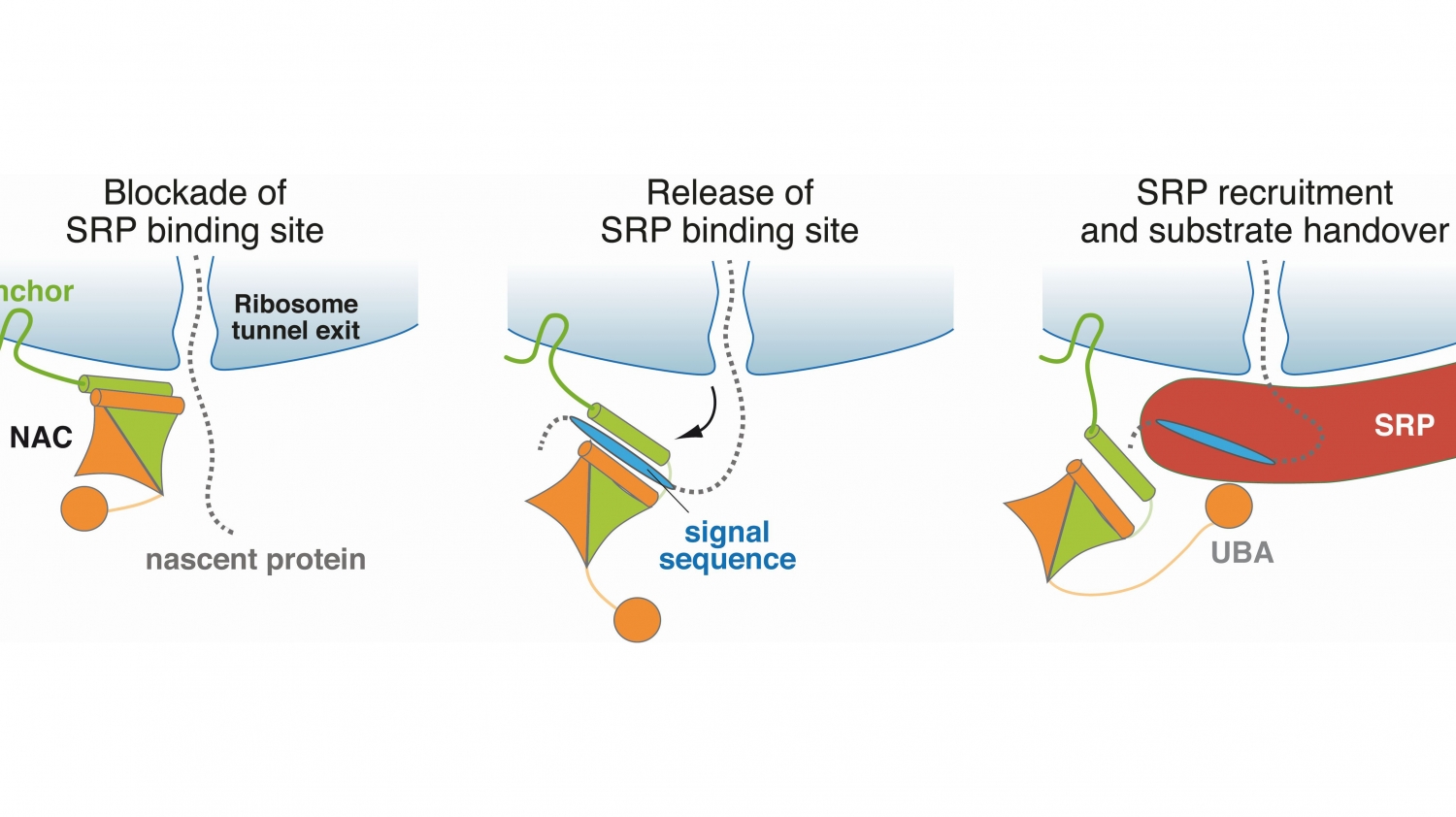
© Martin Gamerdinger, University of KonstanzNAC regulates the transport of proteins within the cell. The figure shows the molecular mechanism underlying this function.
"Although the protein complex NAC was discovered about 30 years ago, its function was virtually unknown until recently. Sophisticated experiments, conducted by the SFB, helped us to explore the function of NAC down to the underlying molecular mechanism."
Elke Deuerling
In two high-profile publications, the researchers showed that NAC has a central and vital control function, as it is involved in both the sorting of newly formed proteins as well as in their modification by methionine excision.
Cultivating the SFB
The SFB scientists were able to make substantial progress and gain knowledge in all areas the projects of the participating biologists and chemists focused on – be it the production of proteins, their modification by chemical groups or protein aggregation and degradation. The previously mentioned examples give just a small glimpse of the SFB's work. "I think the SFB has made an important contribution to proteostasis research. We have gained essential insights into proteostasis, and also developed great ideas for future research projects", Deuerling summarizes. The SFB provided a strong foundation for the future.
"The team spirit, know-how and infrastructure, such as our core facilities with highly specialized equipment, were already here before 2012. For the SFB and our scientific questions, however, it was essential to constantly renew, improve and expand upon this great basis. This also is true of our team. In the past twelve years, we have succeeded in inspiring and recruiting outstanding early career researchers, who have immensely enriched the SFB and research at the University of Konstan.z"
Elke Deuerling
Broader horizons
When the SFB comes to an end in December 2023 – after the longest possible funding period – it is important to look ahead and develop fresh perspectives. Through the SFB, the chemists and biologists from Konstanz have created and expanded the knowledge, methods and structures on which they can now base future large-scale projects. Their clear goal: to build on the strong synergies that have grown and take proteostasis research in Konstanz to the next level. The scientists are ready to get right to work on their new ideas. Erika Isono explains one idea that builds directly on existing know-how: "We would like to focus more on how cells regulate proteostasis in response to mechanical or chemical stress".
For the future, the researchers also want to expand their horizon beyond the protein level and look at the big picture. Even though the regulation of the proteome is undoubtedly one of the most fundamental and vital processes at the cellular level, it is also influenced by other cellular molecules and networks, such as nucleic acids networks. To put it simply: the SFB researchers have, so far, primarily investigated the two-dimensional interconnection of processes at the protein level.
"Therefore, the next step will be including the third dimension in our research and looking at how the protein level is interconnected with other fundamental levels, such as that of nucleic acids, and how biochemical communication runs in this three-dimensional network. This is our next big task, and we are ready for it thanks to our work in the SFB and the great approaches we were able to develop", concludes Deuerling.